Rwanda has been stepping up measures to combat Rift Valley Fever (RVF) following the country’s second outbreak in two years, amid concerns it could spread to humans.
The re-emergence of the disease among livestock, near the border with Tanzania, sparked fears of a repeat of the 2022 outbreak, in which more than 20 people and hundreds of animals died.
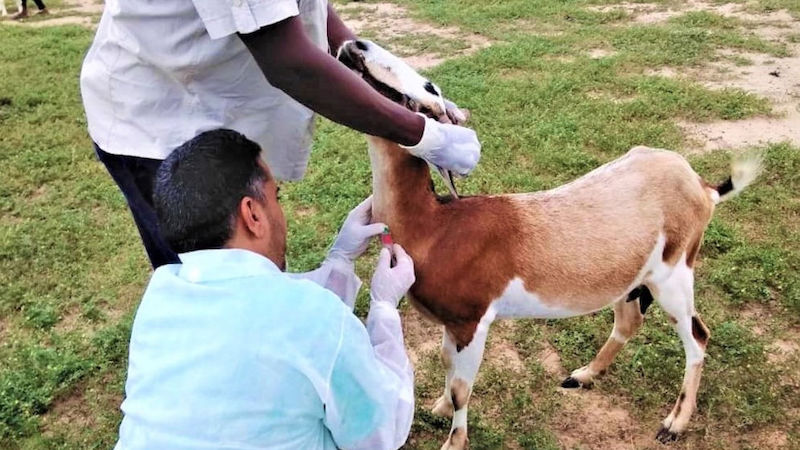
Since the start of the latest outbreak in mid-August, authorities have stepped up surveillance and launched a vaccination campaign for livestock.
However, the absence of rapid diagnostic tests remains a major challenge, disease specialists told SciDev.Net.
What is Rift Valley Fever?
RVF is a viral disease that primarily affects animals but can also infect humans. No human-to-human transmission has been reported to date.
The virus is spread through the bites of infected mosquitoes and through contact with the blood or organs of infected animals. This includes consuming raw meat or unpasteurised milk from infected animals.
In its mild form, humans experience mild, flu-like fever symptoms, or even no symptoms at all. But a small percentage of people affected develop a more severe form, which can result in eye disease and life-threatening conditions such as haemorrhagic fever.
The 2022 outbreak
In 2022, Rwanda experienced its first major RVF outbreak, resulting in 173 human cases and 22 deaths, according to a recent study of the outbreak.
The investigation revealed that delayed screening and testing may have contributed to the spread of the disease.
The findings highlight the urgent need for rapid and accurate diagnostic tools, says Edson Rwagasore, co-author of the study and division manager for public health surveillance and emergency preparedness and response at the Rwanda Biomedical Centre.
“We need rapid tests to deliver instant examination results,” Rwagasore told SciDev.Net.
“We use Polymerase Chain Reaction (PCR) to test for the RVF,” he said, adding: “It takes up to six hours to deliver the results of tests. We have been looking for rapid test kits [but] they are unavailable on the market.”
Race against time
The current outbreak triggered a swift response from Rwandan health authorities, employing a one health approach that integrates human and animal health interventions.
Julien Niyingabira, head of communication at the health ministry, told SciDev.Net: “There is an ongoing testing of people screened for coming into contact with infected animals or their products to determine if they are not positive with the Rift Valley Fever.”
Niyingabira added that over 100 individuals have been tested using PCR, and none have shown positive so far.
However, Rwagasore told SciDev.Net that a lack of rapid test kits was hampering efforts for early detection and timely treatment.
Vaccination efforts
In addition to human surveillance, authorities have launched a vaccination campaign targeting livestock in affected areas.
As of 30 September, 28 positive cases had been identified in livestock, according to the Rwanda Biomedical Centre.
“So far, 8,410 of 32,999 (25 per cent) of earmarked livestock in the area have been vaccinated against RVF in an exercise that started on 7 September, 2024,” reports Fabrice Ndayisenga, who heads the Animal Resources Research department at the Rwanda Agriculture Board.
There is currently no licensed vaccine for humans, but a promising human RVF vaccine candidate, ChAdOx1 RVF, entered Phase II human trials in Kenya earlier this month (13 October).
This marks a significant step towards developing a vaccine to protect vulnerable populations.
Developed on the University of Oxford’s ChAdOx1 vaccine platform—the same technology behind the Oxford-AstraZeneca COVID-19 vaccine—ChAdOx1 RVF has already shown positive results in the first stage of human testing conducted in the UK earlier this year.
“The launch of a Phase II clinical trial of a Rift Valley fever vaccine candidate in an endemic country is a crucial milestone in our efforts to control this disease,” said Jean Kaseya, director general of Africa Centres for Disease Control, when the new trial was launched.
“The ChAdOx1 RVF vaccine offers hope to vulnerable populations who are disproportionately affected by the growing impact of climate change,” he said.
Looking ahead
While the current outbreak is contained, the risk of future outbreaks looms large. Experts emphasise the need for continued vigilance, enhanced surveillance, and investment in research and development.
“We need analytical skills in bioinformatics, the analytical part that leads to accurate scientific conclusions,” said Rwagasore, speaking about the need for capacity building in genomic sequencing analysis to better understand and track the virus.
He said the international community supports research and development of RVF diagnostics, vaccines, and therapeutics through collaboration and information sharing among countries.
This, he says, is crucial to combat this recurring threat effectively.
This piece was produced by SciDev.Net’s Sub-Saharan Africa English desk.
